A new model suggests that as many as 4.8 million symptomatic cases of respiratory syncytial virus (RSV) occur in the United States among patients 65 and older. That burden is greater than previously recognized, according to another study, which looked at the U.S. and other high-income countries. Recently approved vaccines are likely to be game-changers.
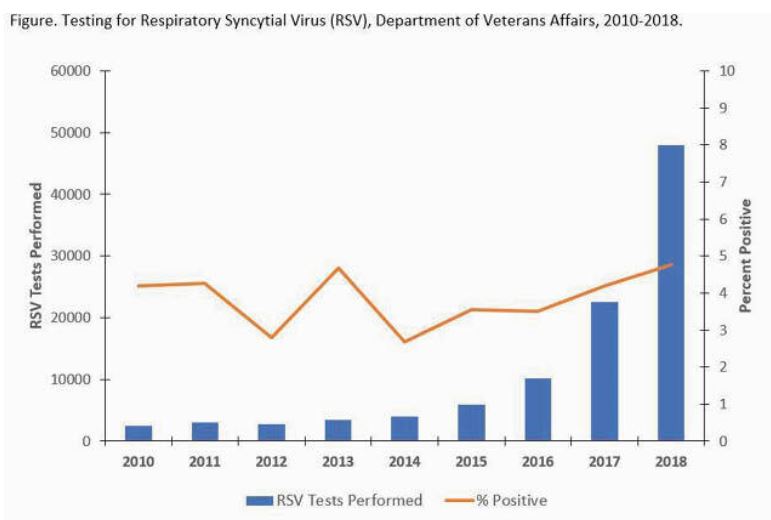
Click to Enlarge: Testing for Respiratory Syncytial Virus (RSV), Department of Veterans Affairs, 2010-2018. Source: Department of Veterans Affairs
PALO ALTO, CA — Respiratory syncytial virus (RSV) disease burden in older adults is greater than previously thought in the United States and other high-income countries, according to industry-funded studies.
At the VA, a 2020 review looking at cases from 2010-2018 found that RSV is an increasingly recognized cause of acute respiratory illness in older adults, which led to an estimated 177,000 hospitalizations and 14,000 deaths each year of the study period.1
Current studies suggested, however, that cases might be undercounted.
Noting that RSV-associated acute respiratory infection (ARI) is an under-recognized cause of illness in older adults, authors of a more-recent report published in the ISIRV conducted a systematic literature review and meta-analysis to better understand the effects.2
The researchers gathered data on RSV-ARI and related hospitalization attack rates and in-hospital case fatality rates (hCFR) in adults 60 or older from the United States, Canada, European countries, Japan and South Korea. To do so, they conducted a systematic literature from Jan. 1, 2000-Nov. 3, 2021, or via other methods (citation search, unpublished studies cited by a previous meta-analysis, gray literature and an RSV-specific abstract booklet). The study team then performed a random effects meta-analysis on estimates from the included studies.
Ultimately, 21 studies were included in the meta-analysis. The researchers determined, based on pooled estimates, the following risks:
- 1.62% (95% confidence interval [CI]: 0.84-3.08) for RSV-ARI attack rate,
- 0.15% (95% CI: 0.09-0.22) for hospitalization attack rate, and
- 7.13% (95% CI: 5.40-9.36) for hCFR.
“In 2019, this would translate into approximately 5.2 million cases, 470,000 hospitalizations, and 33,000 in-hospital deaths in ≥60-year-old adults in high-income countries,” the authors wrote.
“RSV disease burden in adults aged ≥60 years in high-income countries is higher than previously estimated, highlighting the need for RSV prophylaxis in this age group,” the researchers concluded.
In early May, the U.S. Food and Drug Administration approved Arexvy, the first respiratory syncytial virus (RSV) vaccine approved for use in the United States. Arexvy is approved for the prevention of lower respiratory tract disease caused by RSV in adults 60 or older.
“Older adults, in particular those with underlying health conditions, such as heart or lung disease or weakened immune systems, are at high risk for severe disease caused by RSV,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research. “Today’s approval of the first RSV vaccine is an important public health achievement to prevent a disease which can be life-threatening and reflects the FDA’s continued commitment to facilitating the development of safe and effective vaccines for use in the United States.”
“Today marks a turning point in our effort to reduce the significant burden of RSV. Arexvy is the first approved RSV vaccine for older adults, expanding GSK’s industry-leading vaccine portfolio, which protects millions of people from infectious diseases each year. Our focus now is to ensure eligible older adults in the US can access the vaccine as quickly as possible and to progress regulatory review in other countries,” said Tony Wood, chief scientific office of GSK, which is launching the vaccine.
In the study that led to FDA approval, the vaccine was shown to significantly reduce the risk of developing RSV-associated lower respiratory tract disease (LRTD) by 82.6% and reduced the risk of developing severe RSV-associated LRTD by 94.1%.
An FDA advisory panel recently recommended that Arexvy and another candidate from Pfizer be approved to protect against RSV in older adults.
The FDA has said that RSV contributes to more than 10,000 deaths a year among people over age 65, plus at least 60,000 hospitalizations.
Another recent industry study, published in Clinical Infectious Diseases, modeled the epidemiology of RSV in the United States, with a special focus on adults 60 and older with and without vaccination.3
“In the absence of a vaccine, we project 17.5-22.6 million symptomatic RSV ARI cases per year in ≥18-year-olds in the U.S., with 3.6-4.8 million/year occurring in ≥60-year-olds, according to the authors. “Modelling indicates that up to 2.0 million symptomatic RSV ARI cases per year could be prevented in ≥60-year-olds with a hypothetical vaccine (70% vaccine efficacy against symptomatic ARI, and 60% vaccine coverage), and up to 0.69 million cases per year can be prevented in the non-vaccinated population, assuming 50% vaccine impact on infectiousness.”
A third study in Infectious Disease and Therapy demonstrates how the burden of RSV in adults older than 60 might be greatly underestimated, at least partly because there are low levels of RSV testing among adults hospitalized for lower respiratory tract infection in the United States.
“Without RSV-specific treatment for adults, testing is uncommon, leading to potential underestimation of RSV incidence in real-world data studies,” according to the researchers who sought to quantify the frequency of RSV testing during LRTI-related hospitalizations of older adults to help interpret incidence estimates.
To do that, they used administrative and billing data for hospitalizations of adults aged ≥ 65 years with a primary or secondary diagnosis of LRTI during the 2016-2019 RSV seasons (October-April), extracting data from the U.S. all-payer Premier Healthcare Database (PHD).
Results indicate that most of the 937 study hospitals performed RSV testing infrequently during LRTI hospitalization. In fact, the median percentage of LRTI hospitalizations with RSV testing was 4.3%, and 78.4% of hospitals performed RSV testing in less than 25% of LRTI-related hospitalizations.
Hospital Type
They add that RSV testing varied by hospital type. Median percentage tested was significantly higher for:
- hospitals with ≥ 200 beds (9.1%) vs. < 200 beds (1.6%),
- for teaching (11.0%) vs. nonteaching (2.5%) hospitals, and
- in urban (7.4%) vs. rural (0.7%) settings.
The authors point out that the median percentage of RSV testing increased over time, from 0.8% to 6.3% between the 2016-17 and 2018-19, seasons.
Authors of the VA studied agreed, noting, “In adult populations, diagnostic testing for RSV has historically been underutilized.”
The researchers from the Palo Alto, CA, VAMC included 102,251 RSV results, finding that, overall, 4,372 (4.3%) specimens from 4,263 unique individuals were positive with a median age of 67 years (range 0-101). Most, 90%, were male.
Another 1,511 patients (35.4%) also had an RSV-coded hospitalization, although RSV type was specified for only 7.8% of positives.
“During 2010-2018 there were 2,522 RSV-coded hospitalizations (median length of stay = 4 days) among 2,444 unique individuals, which included 413 ICU stays (16.4%) and 98 deaths (3.9%) during the RSV-coded hospitalization,” the researchers pointed out. “Approximately 78% of RSV-coded hospitalizations within VA (excluding all non-VA hospitalizations) had a documented positive test result.”
The study found a greater than 15-fold increase in RSV tests performed, although the percent testing positive remained relatively stable
“RSV testing and identification of patients with RSV infection increased dramatically during the time period analyzed, likely due to increased availability of PCR-based multi-pathogen panels and duplex assays,” the VA authors concluded. “While the percentage of tests positive for RSV remained relatively stable, the rise in coded hospitalizations may be due to increased testing for RSV among hospitalized veterans with severe respiratory infections. These surveillance data may allow for further characterization of RSV disease burden estimates, which can help inform clinical management and development of interventions for adults, such as vaccines and antiviral therapies.”
- Lucero-Obusan C, Schirmer P, Oda G, Holodniy M. 1718. Respiratory Syncytial Virus (RSV) Surveillance in the Department of Veterans Affairs (VA), 2010-2018. Open Forum Infect Dis. 2020 Dec 31;7(Suppl 1):S843. doi: 10.1093/ofid/ofaa439.1896. PMCID: PMC7778290.
- Savic M, Penders Y, Shi T, Branche A, Pirçon JY. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: A systematic literature review and meta-analysis. Influenza Other Respir Viruses. 2023 Jan;17(1):e13031. doi: 10.1111/irv.13031. Epub 2022 Nov 11. PMID: 36369772; PMCID: PMC9835463.
- Van Effelterre T, Hens N, White LJ, Gravenstein S, et. al. Modeling Respiratory Syncytial Virus Adult Vaccination in the United States with a Dynamic Transmission Model. Clin Infect Dis. 2023 Mar 23:ciad161. doi: 10.1093/cid/ciad161. Epub ahead of print. PMID: 36949605.
- Rozenbaum MH, Judy J, Tran D, Yacisin K, Kurosky SK, Begier E. Low Levels of RSV Testing Among Adults Hospitalized for Lower Respiratory Tract Infection in the United States. Infect Dis Ther. 2023 Feb;12(2):677-685. doi: 10.1007/s40121-023-00758-5. Epub 2023 Jan 27. PMID: 36707466; PMCID: PMC9883084.
