Disappointing HITCH Trial Was Halted When Outcomes Weren’t Met
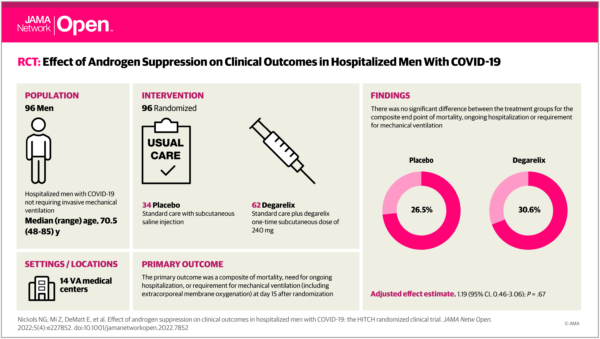
Click to Enlarge: Visual Abstract. Effect of Androgen Suppression on Clinical Outcomes in Hospitalized Men With COVID-19
LOS ANGELES — A potential protective role for suppression of the androgen receptor (AR) against COVID-19 incidence and severity has been observed in some retrospective analyses.
While there was some inconsistency, according to a new report in JAMA Network Open, clinical trials of AR antagonists in the outpatient setting demonstrated accelerated viral clearance and reduced rates of hospitalization.1,2
Optimism about those trials led to the Hormonal Intervention for the Treatment of Veterans With COVID-19 Requiring Hospitalization (HITCH) Phase 2, placebo-controlled, double-blind, randomized clinical trial. VA Greater Los Angeles Healthcare System-led researchers compared the efficacy of degarelix plus standard care vs. placebo plus standard care on clinical outcomes in men hospitalized with COVID-19 but not requiring invasive mechanical ventilation.
Degarelix is approved by the Food and Drug Administration (FDA) for prostate cancer therapy. With an immediate onset of action, the drug binds to the gonadotropin-releasing hormone receptors in the pituitary gland and rapidly suppresses luteinizing and follicle-stimulating hormone secretion. That decreases testosterone production within the testes, according to the study, and rapidly reduces circulating androgen levels.
The study team, including representatives from VAMCs in Perry Point, MD; Albuquerque, NM; Charleston, SC; Little Rock, AR; Long Beach, CA; New York; Brooklyn, NY; Memphis, TN; Seattle, WA; Philadelphia; Dallas; Houston; Phoenix, AZ, and St. Louis, MO, as well as academic medical centers, enrolled in-patients at 14 VA hospitals from July 22, 2020, to April 8, 2021. Data were analyzed from Aug. 9 to Oct. 15, 2021.
The trial was undertaken because SARS-CoV-2 entry requires the TMPRSS2 cell surface protease, and antiandrogen therapies reduce expression of TMPRSS2. Researchers sought to determine whether temporary androgen suppression induced by degarelix improved the clinical outcomes of COVID-19 inpatients.
The randomized clinical trial, which included 96 men, was stopped for futility, however, when androgen suppression with the addition of degarelix vs. placebo plus standard care did not result in lower rates of mortality, ongoing hospitalization or requirement for mechanical ventilation—all part of the composite endpoint.
For the study, patients had been stratified by age, history of hypertension and disease severity. Participants were centrally randomized 2:1 to a one-time subcutaneous dose of 240 mg of degarelix or a saline placebo. Researchers advise that standard care included but was not limited to supplemental oxygen, antibiotics, vasopressor support, peritoneal dialysis or hemodialysis, intravenous fluids, remdesivir, convalescent plasma and dexamethasone.
Trial Was Halted
When the trial was halted after the planned interim analysis, there were 96 evaluable patients, including 62 patients randomized to the degarelix group and 34 patients in the placebo group, out of 198 initially planned. Participants had a median age of 70.5 and common comorbidities including:
- chronic obstructive pulmonary disorder (15 patients [15.6%]),
- hypertension (75 patients [78.1%]),
- cardiovascular disease (27 patients [28.1%]),
- asthma (12 patients [12.5%]),
- diabetes (49 patients [51.0%]), and
- chronic respiratory failure requiring supplemental oxygen at baseline prior to COVID-19 (9 patients [9.4%]).
Researchers identified no significant difference between the degarelix and placebo groups (19 patients [30.6%] vs. 9 patients [26.5%]; P =0 .67) for the primary end point. The same was true for any secondary endpoints, including inpatient mortality (11 patients [17.7%] vs. 6 patients [17.6%]) or all-cause mortality (11 patients [17.7%] vs. 7 patents [20.6%]).
The study also found no differences between degarelix and placebo groups in the overall rates of adverse events (13 patients [21.0%] vs. 8 patients [23.5%) and serious adverse events (19 patients [30.6%] vs. 13 patients [32.4%]), nor unexpected safety concerns.
“In this randomized clinical trial of androgen suppression vs placebo and usual care for men hospitalized with COVID-19, degarelix did not result in amelioration of COVID-19 severity,” the study concluded.
The authors suggest the following reasons the trial did not go as anticipated:
- First, suppression of testosterone is itself a physiologic response to acute, critical illness. In the HITCH study, total testosterone decreased in both treatment groups, though markedly more so in the degarelix group. “It is plausible that the reduction of testosterone in the placebo group in response to COVID-19 illness reached a threshold to affect physiology. Accordingly, the additional testosterone reduction by degarelix may not have yielded further inhibition of viral coreceptor expression, although we did not directly analyze TMPRSS2 or ACE2 expression in this trial,” the researchers pointed out.
- Second, the timing of downregulation of TMPRSS2 may have limited the effect of androgen deprivation therapy on the severity of illness. At enrollment, patients might no longer have been dependent on ongoing viral infection, but rather on a hyperactivated immune response that results in end-organ damage. “If this were the case, then reduction of the expression of the viral coreceptors would not be expected to have had an impact on the course of the disease” the authors posited.
- Third, because androgens are immunosuppressive, they might have inhibited innate and adaptive immunity, including T-cells. “As such, heightened suppression of serum testosterone could have further activated an already hyperactivated immune system, which could counter any beneficial effect that may have been mediated by suppression of viral coreceptors,” according to the study.
- Fourth, in men, serum estrogen is derived from testosterone in men, so that testosterone suppression should result in lower absolute concentrations of serum estrogen, which has been reported to suppress the expression of viral coreceptors. “In the context of medical castration, reduced estrogen concentrations could counterbalance the effects of reduced testosterone on viral coreceptor expression,” the authors wrote.
- Finally, despite preclinical data, “it is also possible that androgens do not regulate TMPRSS2 and ACE2 in relevant tissues to an extent that is targetable by antiandrogen therapy to ameliorate severity of COVID-19 in patients,” the researchers stated, suggesting that earlier use of androgen-directed therapies during the initial infection and viral replication might have been a more effective strategy.
- McCoy J, Goren A, Cadegiani FA, et al. Proxalutamide reduces the rate of hospitalization for COVID-19 male outpatients: a randomized double-blinded placebo-controlled trial. Front Med (Lausanne). 2021;8:668698. doi:10.3389/fmed.2021.668698PubMedGoogle Scholar
- Cadegiani FA, McCoy J, Gustavo Wambier C, et al. Proxalutamide significantly accelerates viral clearance and reduces time to clinical remission in patients with mild to moderate COVID-19: results from a randomized, double-blinded, placebo-controlled trial. Cureus. 2021;13(2):e13492. doi:10.7759/cureus.13492PubMedGoogle Scholar
- Nickols NG, Mi Z, DeMatt E, et al. Effect of Androgen Suppression on Clinical Outcomes in Hospitalized Men With COVID-19: The HITCH Randomized Clinical Trial. JAMA Netw Open. 2022;5(4):e227852. doi:10.1001/jamanetworkopen.2022.7852
