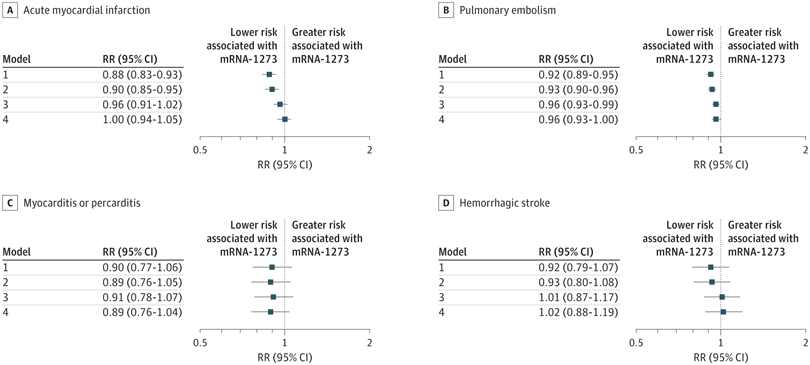
Click to Enlarge:A to D, Risk ratios (RRs) and 95% CIs for acute myocardial infarction (A), pulmonary embolism (B), myocarditis or pericarditis (C), and hemorrhagic stroke (D) were estimated using generalized linear models with a binominal distribution and log link function. Model 1 was unadjusted; model 2 was adjusted for region and month of vaccination; model 3 was adjusted for age, sex, race and ethnicity, and frailty; and model 4 was adjusted for region, month of vaccination, age, sex, race and ethnicity, frailty, claim source (eg, pharmacy and/or Medicare), time since prior documented COVID-19 infection, time since prior hospitalization, time since prior outpatient visit, and time since prior emergency department visit. Risk ratios are interpreted as the relative difference in the outcome between mRNA-1273 vs BNT162b2, whereby an RR of 1.00 represents no relative difference in risk. Source: JAMA Network Open
PROVIDENCE, RI — Receipt of the Moderna COVID-19 vaccine by older adults was associated with a lower risk of adverse events than the Pfizer-BioNTech version, according to a new study.
Researchers from Brown University, the Providence, RI, VAMC and colleagues suggested that one possible reason was improved protection against SARS-CoV-2.
Their study sought to determine whether there were any safety differences between mRNA vaccines for COVID-19 and whether those differences varied by frailty levels. Results were published in JAMA Network Open.1
The cohort study of about 6.4 million older U.S. adults found a 4% lower risk of pulmonary embolism, a 2% lower risk of thromboembolic events and a 14% lower risk of diagnosed COVID-19 among those who received the mRNA-1273 vaccine compared with the BNT162b2 vaccine.
“Although both vaccines were safe across frailty subgroups, differences were generally greater in individuals without frailty,” the researchers advised.
The researchers pointed out, “Head-to-head safety comparisons of the mRNA vaccines for SARS-CoV-2 are needed for decision-making; however, current evidence generalizes poorly to older adults, lacks sufficient adjustment and inadequately captures events shortly after vaccination. Additionally, no studies to date have explored potential variation in comparative vaccine safety across subgroups with frailty or an increased risk of adverse events, information that would be useful for tailoring clinical decisions.”
The retrospective cohort study was conducted between Dec. 11, 2020, and July 11, 2021, with 28 days of follow-up following the week of vaccination. Data analysis began on Oct. 18, 2022.
The participant group represented more than 50% of the U.S. Medicare population, and information was pulled from a novel linked database of community pharmacy and Medicare claims data. The focus was on community-dwelling, fee-for-service beneficiaries aged 66 years or older who received mRNA-1273 vs. BNT162b2 as their first COVID-19 vaccine. The mean (SD) age was 76.3, with 59.4% being female and 86.5% being white. Of the study group, 38.1% were categorized as prefrail and 6.0% as frail.
The study team assessed 12 potential adverse events, such as pulmonary embolism, thrombocytopenia purpura and myocarditis, individually. Frailty was measured using a claims-based frailty index, with beneficiaries being categorized as nonfrail, prefrail and frail. The risk of diagnosed COVID-19 was assessed as a secondary outcome.
Low Adverse Effects Rate
Results indicated that the risk of all outcomes was low in both vaccine groups. In adjusted models, the mRNA-1273 vaccine was associated with a lower risk of pulmonary embolism (RR, 0.96 [95% CI, 0.93-1.00]; RD, 9 [95% CI, 1-16] events per 100,000 persons) and other adverse events in subgroup analyses (eg, 11.0% lower risk of thrombocytopenia purpura among individuals categorized as nonfrail).
“The mRNA-1273 vaccine was also associated with a lower risk of diagnosed COVID-19 (RR, 0.86 [95% CI, 0.83-0.87]), a benefit that was attenuated by frailty level (frail: RR, 0.94 [95% CI, 0.89-0.99]),” the authors pointed out.
Background information in the article noted that, as of January 2023, about 70% of the global population has received at least one COVID-19 vaccine, with the BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) messenger RNA (mRNA) vaccines being among the most widely used. Public health officials recommended them because of evidence of their superior safety and efficacy relative to other products.
Yet, the authors wrote, “Few studies have directly compared the risk of potential adverse events between mRNA vaccines, which differ in their manufacturing, administration and immune response. Existing head-to-head comparisons of BNT162b2 and mRNA-1273 have shown small yet potentially meaningful differences in the risk of several adverse events that can vary by age and sex. However, current estimates generalize poorly to older adults and are derived from samples that are too small to capture rare events over a short and clinically relevant follow-up period.”
In addition, they said, no studies had assessed comparative vaccine safety “within and across patient subgroups with increased frailty or history of the diagnoses identified as vaccine-associated adverse events—conditions likely to modify vaccine response and potentially contribute to differences in safety.”
Confusing the issue is that several of the potential vaccine-associated adverse events are also sequelae of SARS-CoV-2. “As previously suggested, a more effective vaccine may appear to be safer for some outcomes due to the enhanced and differential prevention of COVID-19,” the study pointed out. “Because of the prevalence of SARS-CoV-2 at the time of early vaccination efforts and observed differences in mRNA vaccine effectiveness, additional studies are needed to understand the extent to which differences in adverse events may be attributed to differential early effectiveness.”
The researchers added that the mRNA-1273 also demonstrated generally larger
“Regardless of the underlying mechanism, however, the comparative reduction in morbidity associated with mRNA-1273 is notable and may have real benefits at the population level,” they wrote. “Nonetheless, studies confirming the extent to which differences in adverse events can be attributed to early effectiveness are needed.”
Those studies should take frailty into account, according to the authors, because the condition “is known to attenuate vaccine response,” adding that “the greater immunogenicity associated with mRNA-1273 may have been diminished in individuals categorized as frail, thereby reducing its degree of differential protection against COVID-19 and its sequelae.”
- Harris DA, Hayes KN, Zullo AR, et al. Comparative Risks of Potential Adverse Events Following COVID-19 mRNA Vaccination Among Older US Adults. JAMA Netw Open. 2023;6(8):e2326852. doi:10.1001/jamanetworkopen.2023.26852

