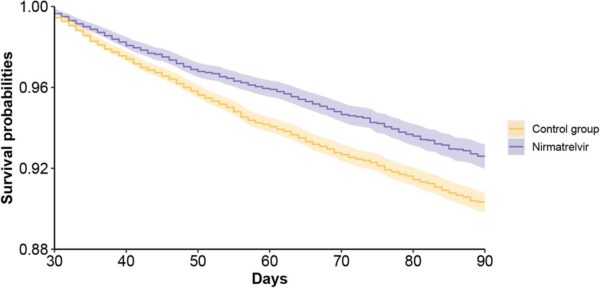
Click to Enlarge: Survival probability of post-acute sequelae in nirmatrelvir and no treatment control group. a. post-acute sequelae of COVID-19 (PASC); Source: MedRXiv
ST. LOUIS — New research from the VA determined that Paxlovid can reduce the risk of symptoms of long COVID by about 25%.
The study, “Nirmatrelvir and the risk of post-acute sequelae of COVID-19,” was released before peer-review on the pre-print server medRxiv. It involved more than 56,000 U.S. veterans with a positive SARS-CoV-2 test.1
Researchers from the VA St. Louis Health Care System and Washington University in St. Louis demonstrated that those patients given the oral antiviral medication Paxlovid in the first five days of a COVID-19 infection had a 25% decreased risk of developing 10 of 12 different Long COVID conditions.
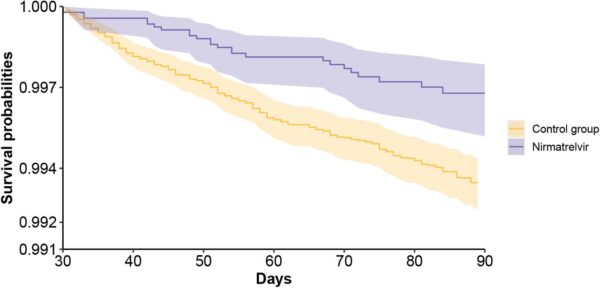
Click to Enlarge: Survival probability of post-acute sequelae in nirmatrelvir and no treatment control group. b. death; Source: MedRXiv
Those conditions included:
- heart disease,
- blood disorders,
- fatigue,
- liver disease,
- kidney disease,
- muscle pain,
- neurocognitive impairment and
- shortness of breath.
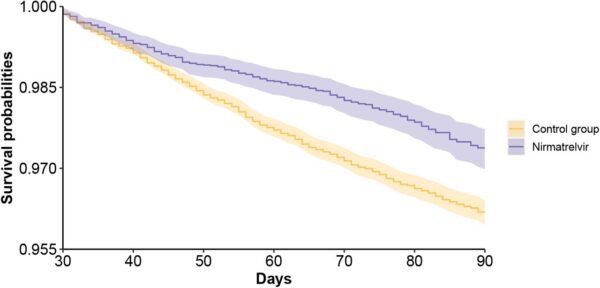
Click to Enlarge: Survival probability of post-acute sequelae in nirmatrelvir and no treatment control group. c. hospitalization; Source: MedRXiv
The authors advised that the decreased risk of long COVID associated with Paxlovid treatment occurred across the board—whether it was a veteran’s first infection or a reinfection and regardless of whether the patient was unvaccinated, vaccinated or boosted.
“Paxlovid reduces the risk of severe COVID-19 in the acute phase, and now we have evidence that it can help reduce the risk of long COVID,” said lead researcher Ziyad Al-Aly, MD, chief of research and development at the VA St. Louis Health Care System and clinical epidemiologist at Washington University in St. Louis. “This treatment could be an important asset to address the serious issue of long COVID.”
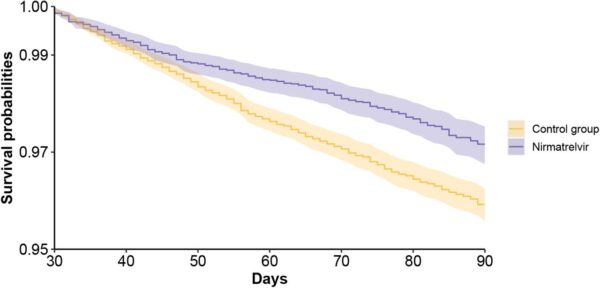
Click to Enlarge: Survival probability of post-acute sequelae in nirmatrelvir and no treatment control group. d. composite outcome of death or hospitalization. Outcomes were ascertained 30 days after the SARS-CoV-2 positive test until the end of follow-up. Survival probability presented for nirmatrelvir (purple, N=9217) and control group (orange, N=47,123). Shaded areas are 95% confidence intervals. Source: MedRXiv
Paxlovid was approved by the Food and Drug Administration in December 2021 for COVID-positive patients at high risk for severe COVID-19. The product is a combination of two medications—nirmatrelvir and ritonavir—and has been shown to lower the risk of hospitalization and death COVID-19 in infected patients.
Results indicated that, compared to the control group, “treatment with nirmatrelvir was associated with reduced risk of [post-acute PASC (HR 0.74 95% CI (0.69, 0.81), ARR 2.32 (1.73, 2.91)) including reduced risk of 10 of 12 post-acute sequelae of SARS-CoV-2] in the cardiovascular system (dysrhythmia and ischemic heart disease), coagulation and hematologic disorders (deep vein thrombosis, and pulmonary embolism), fatigue, liver disease, acute kidney disease, muscle pain, neurocognitive impairment and shortness of breath. Nirmatrelvir also was associated with reduced risk of post-acute death (HR 0.52 (0.35, 0.77), ARR 0.28 (0.14, 0.41)), and post-acute hospitalization (HR 0.70 (0.61, 0.80), ARR 1.09 (0.72, 1.46)).”
‘Groundbreaking Study’
“This groundbreaking study is going to improve the lives of veterans and all Americans,” said VA Secretary Denis McDonough. “VA’s researchers have conducted life-saving studies throughout the pandemic, and Dr. Al-Aly’s excellent work here is yet another example of VA leading the way.”
Background information in the article noted that long COVID affects millions of people worldwide. “Prevention of PASC is an urgent public health priority,” the authors emphasized.
In sum, the researchers concluded, “our results show that in people with SARS-CoV-2 infection who had at least 1 risk factor for progression to severe COVID-19 illness, treatment with nirmatrelvir within 5 days of a positive SARS-CoV-2 test was associated with reduced risk of PASC regardless of vaccination status and history of prior infection. The totality of findings suggests that treatment with nirmatrelvir during the acute phase of COVID-19 reduces the risk of post-acute adverse health outcomes.”
Another recent study led by Aly pointed out that an estimated 10% to 30% of people who survive the acute infection have to endure long COVID. In their report in Nature Medicine, concern was raised about the numbers of vaccinated people are being diagnosed with breakthrough infections and whether those patients also experienced post-acute sequelae.2
Their study of more than 13 million veterans found that long COVID occurs with breakthrough infections, but at a slightly reduced rate compared to those who are unvaccinated.
In that study, researchers analyzed the deidentified medical records of more than 13 million veterans in VA national healthcare databases. They examined data of 113,474 unvaccinated COVID-19 patients and 33,940 vaccinated patients who had experienced COVID-19 breakthrough infections, all from Jan. 1 through Oct. 31, 2021.1
“Initially what we tried to determine is if people with breakthrough infections get long COVID at all, and what we found was that indeed they do,” Al-Aly, MD, told U.S. Medicine. The study team found that vaccination reduced the risk of long COVID, but only by 15%. “So there was some reduction, but it wasn’t huge,” he said.
Other findings of the study showed some greater benefits for vaccinated individuals, however, he said. “When you look deeper into the different results, the reduction was quite significant for two key areas—respiratory problems and coagulation disorders. Vaccination reduces risk of lung problems and also blood clotting by 50%.” The researchers noted that vaccination against SARS-CoV-2 reduced the risk of death by 34%.
Other findings of the study included:
- Long COVID risks were 17% higher among vaccinated immunocompromised people with breakthrough infections compared with previously healthy, vaccinated people who experienced breakthrough infections.
- Vaccinated patients who were hospitalized with breakthrough COVID-19 infections experienced 2.5 times the risk of death than people who were hospitalized with influenza. They also had a 27% higher risk of long COVID in the first 30 days after diagnosis, compared with 14,337 people who were hospitalized with seasonal influenza.
- Across the board, people who had breakthrough COVID-19 faced significantly higher risks of death and illnesses, such as heart and lung diseases, neurological conditions and kidney failure compared to long-term health outcomes with a pre-pandemic control group of more than 5.75 million people.
- Xie Y, Choi T, Al-Aly Z. Nirmatrelvir and the Risk of Post-Acute Sequelae of COVID-19. medRxiv 2022.11.03.22281783; doi: https://doi.org/10.1101/2022.11.03.22281783
- Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022 May 25. doi: 10.1038/s41591-022-01840-0. Epub ahead of print. PMID: 35614233.
